People’s Republic of China (PRC) vaccine management law, you can be more perfect.
Last night, I was lying in bed, swiping the screen to complete the last purchase of the Double Eleven, and I was caught off guard by the fans that the draft of People’s Republic of China (PRC) Vaccine Management Law (hereinafter referred to as "Vaccine Law") was released.
This morning, many friends of media reporters call me on WeChat and asked me to express my views. I read the draft for comments carefully and gave the following 14 points.
Viewpoint 1: The vaccine law is higher than the vaccine regulations and more comprehensive.
Previously, the most authoritative normative document in the field of vaccines was the State Council Order No.668 promulgated in 2005 and revised in 2016-Regulations on the Administration of Vaccine Circulation and Vaccination (hereinafter referred to as "Vaccine Regulations"). After consulting a senior legal person, I learned that if the vaccine law is formally promulgated, it should be passed by the National People’s Congress and signed and promulgated by president, and its status is higher than the vaccine regulations.
As a practitioner in the field of vaccines, my ass determines my head. I support our country to regulate the production, circulation and vaccination of vaccines with proprietary laws on the basis of the existing drug administration law. This shows that the country attaches great importance to the vaccine as a special drug and public health, and it is a great opportunity for the development of the vaccine field. As a vaccine person, I also feel a great responsibility.
Both the Vaccine Regulations and the Vaccine Law regulate the two fields of vaccine circulation and vaccination. The Vaccine Law also puts forward many new requirements for production enterprises in the second to fourth chapters, and the ninth and tenth chapters also strengthen the definition of supervision and management and legal responsibility.
In the full text of the vaccine law, there is no mention of the relationship with the vaccine regulations. I wonder if it is an omission? According to my understanding, it should be that once the vaccine law is implemented, the vaccine regulations should be abolished.
Viewpoint 2: Vaccines are related to national security.
Article 1 [Legislative Purpose] This Law is formulated to ensure the safety, effectiveness and accessibility of vaccines, standardize vaccination, safeguard and promote public health and safeguard national security.
The first article of the Vaccine Law links vaccines with national security, which is an unprecedented formulation.
In fact, it is not surprising that vaccines mainly prevent infectious diseases.
Like smallpox, a deadly infectious disease of the reaper, it was eliminated by vaccination in the last century. Polio is also a disease that seriously endangers children’s health. At present, it has been eliminated by vaccination against polio in China. The next disease to be eliminated should be measles.
However, there is also an infectious disease that poses a great threat to life-influenza, and its vaccination rate in China is less than 2%. If a mutated influenza virus causes a global epidemic of plague, such vaccination rate can’t stop the pace of death at all. Especially this year, due to various accidents and poor coordination, the supply of influenza vaccine in China has been reduced by more than half compared with the same period of last year.
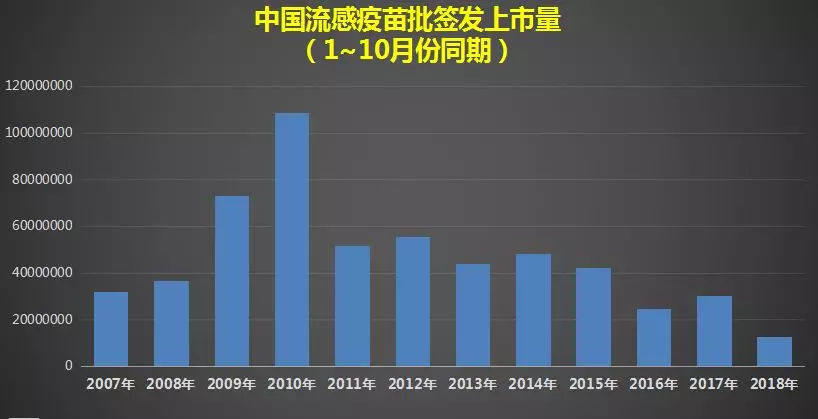
If an influenza pandemic really occurs this year, the serious shortage of influenza vaccine will lead to the epidemic situation being uncontrollable, and public health and the national economy are likely to be hit hard.
Therefore, linking vaccines with national security is by no means alarmist.
Viewpoint 3: Relevant definitions and terms need to be clarified.
Article 2 [Scope of Application] The term "vaccine" as mentioned in this Law refers to preventive biological products used for human immunization in order to prevent and control the occurrence and epidemic of diseases.
At the beginning of the formulation and revision of the Vaccine Regulations, vaccines were defined as: …… preventive biological products of vaccines. As you can see, there has been a phenomenon of using "vaccine" to explain vaccines, which is really inappropriate.
The definition of vaccine in vaccine law avoids the embarrassment of circular explanation, but this definition is still not perfect.
Are there only vaccines for preventive biological products? Actually, it is not.
China is a big country of hepatitis B. For babies whose mothers are carriers of hepatitis B, our strategy to prevent them from being infected with hepatitis B is to vaccinate them with hepatitis B vaccine and hepatitis B immunoglobulin. Hepatitis B immunoglobulin also meets the definition of vaccine in the vaccine law here, but it is not a vaccine.
The essence of vaccine is to make the human body actively produce immunity against diseases, and its purpose is prevention for the time being, and it is likely to include treatment in the future.
Vaccines in the Vaccine Law should refer to vaccines for preventive use, so its definition is suggested to be changed to: …… preventive biological products used for human body to actively generate immunity.
The term [immunization] also appears in the definition of vaccine, and the other parts of the full text use [vaccination], so it is suggested to unify it into a more popular [vaccination]. In the full text, it is also suggested that [immunization program] be changed to [vaccination program] in a unified way.
Viewpoint 4: Vaccine classification should be simple and clear.
Article 2 [Scope of Application] Vaccines are divided into immunization programs and non-immunization programs.
Vaccines are divided into the first category and the second category in the vaccine regulations, and it is necessary to explain to the public what are the first and second categories. The new classification of vaccine law essentially corresponds to the second classification in the vaccine regulations, but it does not reduce the difficulty of understanding. It is necessary to explain what immunization planning is at the end of the full text.
China CDC has a National Immunization Planning Center. If vaccines are divided into immunization vaccines and non-immunization vaccines, then does the National Immunization Planning Center manage non-immunization vaccines?
In fact, whether it is the first type of vaccine/the second type of vaccine, or the immunization program vaccine/the non-immunization program vaccine, the vernacular is free vaccine/self-funded vaccine.
I strongly recommend that vaccine classification return to its essence and use the free vaccine/self-funded vaccine classification that can be seen by the public at a glance. Free vaccines can also be divided into: national free vaccines and local free vaccines (this concept corresponds to the national immunization program vaccine and other immunization programs vaccines in Article 36).
Viewpoint 5: Reward whistleblowers and avoid small evils from forming big evils.
Article 12 [Social Co-governance] Any organization or individual has the right to report illegal acts of vaccines, get information about vaccines from relevant departments according to law, and put forward opinions and suggestions on vaccine supervision and management.
This time, there was a serious violation of regulations in the production process of Changchun Changsheng vaccine. It is said that after an insider reported it, the flight inspection of the relevant state departments finally confirmed the problem. However, its violation has existed for four years, and it has not been discovered during this period.
In my opinion, China should establish a system of rewarding whistleblowers (also called whistleblower system), and use 10% of the fine to reward whistleblowers, and publicize the rewards, but protect their privacy. Only in this way can enterprises be conscientious, abide by all kinds of rules and regulations on thin ice, and avoid doing evil from the source. Or once there is a small evil, it will be reported by insiders to avoid a small evil like Changchun Changsheng to develop a big evil.
Although China’s traditional culture does not encourage reporting, it is through reporting that vicious incidents like Changchun Changsheng were discovered. If there is no reporting system, many corrupt officials and moths will always be at large. In my opinion, as long as it is beneficial to the public interest, the report of corporate evil should be explicitly encouraged by the government, and the way to encourage it is to reward it heavily.
Viewpoint 6: Publicize the details in batches and give full play to the advantages of big data governance (the most important viewpoint of this article).
Twenty-fifth [vaccine batch issuance] batch issuing institutions shall publish the results of vaccine batch issuance in a timely manner for public inquiry.
The publication of batch issuance is very, very, very critical. However, if we only publicize whether the vaccine has been issued in batches, it is a step in the same place and does not jump out of the thinking mode of piecemeal treatment.
In order to ensure the quality of the vaccine and realize the survival of the fittest, I strongly suggest that the detailed test report of the vaccine, that is, the test value of each index (as detailed as the routine blood report), be publicized.
At present, most vaccines in China can be issued in batches. However, the indicators of vaccines issued in batches are also good or bad, some just pass the exam, and some are excellent. Who doesn’t want to get a vaccine with excellent indicators and appropriate prices?
As long as the batch inspection report of each batch of vaccines can be publicized, we can use the big data of thousands of batches of vaccine inspection reports every year to score each vaccine product of each manufacturer based on a scoring system, and the results can be provided to the public through WeChat applet for free inquiry.
When parents take their children to vaccinate, it will be no longer difficult to choose vaccines of various brands. Just take out your mobile phone and check the score calmly, and then make the most rational choice in combination with its price. A vaccine product with no price advantage and the lowest score will only be eliminated if it does not strive to improve the quality and reduce the cost.
People who often fly will use Fei Changzhun’s mobile APP. Among them, there are the punctuality data of major airlines’ flights. If the prices are similar, then passengers will definitely choose to avoid flights with low punctuality, right?
Through thorough data disclosure, we can play the invisible hand of market economy and force vaccine manufacturers to regard vaccine quality as their lifeline. In the past, enterprises passively accepted supervision, but now enterprises actively improve vaccine quality and reduce production costs in order to survive, which naturally improves the overall vaccine quality level in China. The regulatory measures and penalties set in the vaccine law to ensure the quality of vaccines are extremely expensive and the effect is difficult to guarantee. Is it really necessary?
Issuing test reports in public does not need to increase the cost (the data is ready-made), but it can greatly improve the level of social governance and reduce management expenses, and can completely become a model of government governance.
I really can’t think of any reason why the government doesn’t publicize the approval of the test report.
Viewpoint 7: Expired vaccines can be recycled through medical waste disposal channels.
Article 39 [Vaccine Supply and Distribution] The holder of the vaccine marketing license shall supply vaccines to provincial disease prevention and control institutions in accordance with the provisions of the procurement contract, and shall not supply vaccines to other units or individuals.
The holder of the vaccine marketing license is responsible for distributing the vaccine to the provincial disease prevention and control institutions, which are responsible for distributing the vaccine to the inoculation units. Vaccine marketing license holders and provincial disease prevention and control institutions shall have the conditions for cold chain storage and transportation of vaccines, or entrust qualified distribution enterprises to distribute vaccines.
In the vaccine regulations, vaccine manufacturers can only supply vaccines at their own expense to county-level CDC (free vaccines are supplied to provincial CDC); In the vaccine law, vaccine manufacturers can only supply vaccines (including free vaccines and vaccines at their own expense) to provincial CDC.
I support this change, because it can make full use of the existing cold chain storage and distribution resources of provincial CDC and reduce the vaccine distribution cost of production enterprises.
However, a loophole that must be filled is that provincial CDC can not only entrust qualified enterprises to distribute vaccines, but also entrust them to store vaccines. All vaccine storage and distribution services are entrusted to enterprises, which provide one-stop service, which is convenient for supervision and can reduce the government’s investment in cold chain hardware for provincial CDC.
If this loophole is not filled, the provincial CDC will build a huge cold storage to store vaccines, and distribution companies often drive several huge refrigerated trucks to the CDC to extract vaccines, which brings inconvenience to the daily management of the CDC. Shanghai CDC has outsourced all storage and distribution services to qualified enterprises, so it is almost impossible and unnecessary to rebuild the cold storage.
It is mentioned in this clause that the provincial CDC is responsible for distributing the vaccine to the inoculation unit. Can the municipal CDC and the county CDC participate in this process? If they can’t participate, the cold chain equipment and managers of these two levels of vaccine storage and distribution will withdraw from the historical stage, which will have a great impact (especially the county-level CDC may have increased the investment in related software and hardware after 2016).
If only the provincial CDC can deliver the vaccine to the inoculation unit, does the provincial CDC have the ability to do it (especially in a vast area)? This must be investigated.
Viewpoint 8: The monitoring of vaccine transportation temperature should be based on science and should not encourage fraud.
Article 42 [Provisions on Vaccine Sales Records] … If the temperature monitoring records of this transportation process cannot be provided or the temperature control does not meet the requirements, it shall not be accepted or purchased …
This clause will make the temperature record of vaccine transportation untrue.
It is difficult to avoid the actual vaccine exceeding 8 degrees during transportation. If a temperature record of 10 degrees is monitored, must such a vaccine be rejected? Is it necessary to be so strict?
Scientifically unnecessary. At present, it has been confirmed that the vaccine can tolerate a certain degree of high temperature exposure, which is beyond the imagination of ordinary people. Continuous exposure at 37 degrees for 12 hours will not lead to unqualified quality of any existing vaccine. All vaccines have been tested for thermal stability before going on the market.
Therefore, although it is necessary to monitor the temperature of vaccine storage, transportation and transportation, vaccines should never be rejected because of one or two overtemperatures, which will only lead to a large number of qualified vaccines being scrapped and a tight supply of vaccines; Or it is caused by the falsification of temperature monitoring records, and all parties are tacitly aware.
I suggest that the state collect the data of vaccine thermal stability test from vaccine manufacturers and make it public. If the vaccine is over-temperature during storage and transportation, it can be compared with the published data to determine whether it needs to be rejected or scrapped.
Electronic temperature monitoring data is still important, which can be used to find out the out-of-control links during storage and transportation, and then improve the temperature control of these links, but it should not be used as the final basis for judging whether the vaccine is rejected/scrapped.
In addition to electronic temperature monitoring, there is a more intuitive solution, which is the vaccine temperature label (VVM) highly recommended by the World Health Organization and UNICEF. By sticking VVM on the vaccine package, the high temperature exposure of the vaccine in the whole circulation process can be recorded, and it can be recognized by users with intuitive colors.
In the picture below, the earthquake and tsunami in Indonesia caused the house of vaccination clinic to be damaged, and the vaccine refrigerator or cold storage was cut off for many days. However, because the vaccines are all affixed with VVM, it is judged by naked eyes that the purple depth of the center square of VVM of OPV exceeds the surrounding purple, and the vaccine must be scrapped. Although the VVM of the other five vaccines also changed color, they did not exceed the limit, and these vaccines can be used. BCG (BCG) was scrapped directly because it was not affixed with VVM.

I think that China should actively promote the use of vaccine VVM. At present, the cost of single chip of VVM is around 0.5 yuan, which is unacceptable for some vaccines. However, VVM can be attached to the packaging of vaccines, that is, every 10 vaccines are attached to the packaging box, and the cost of a single vaccine is only 0.05 yuan.
With VVM, it is possible to judge whether it is necessary to reject/scrap vaccines by looking at VVM in the process of vaccine delivery and handover. VVM can filter out most of the occasional temperature exceeding the standard, and electronic temperature monitoring records can present real records, which can promote us to find the weak links of temperature control and improve them.
With VVM, the recipients can also intuitively see the temperature exposure of the vaccine from production to inoculation on themselves, without consulting the complicated electronic temperature monitoring records, so as to make the recipients feel at ease to inoculate the vaccine.
Viewpoint 9: Expired vaccines can be recycled through medical waste disposal channels.
Article 43 [Destruction of Expired Vaccines] … Expired vaccines shall be uniformly registered and recovered by county-level disease prevention and control institutions …
For expired vaccines, management should be strengthened. However, the practice of recycling by county-level CDC seems professional, but it is the old road of closed management.
Any inoculation unit must be a medical institution, and medical institutions must implement the Regulations on the Management of Medical Wastes. Compared with various infectious wastes produced in clinic, expired vaccines are safer wastes. Expired vaccines can be treated in time through the medical waste disposal channels of medical institutions, and can be recycled by county-level CDC without hoarding.
It is impossible for county-level CDC to recycle expired vaccines in real time, and the expired vaccines of vaccination units must be hoarded for a period of time before being recycled. The possibility of misuse cannot be ruled out by hoarding expired vaccines, so seemingly professional regulations will increase the risk of misuse of expired vaccines.
It is suggested that this article be changed to: Expired vaccines should be handed over to the medical waste disposal channels of medical institutions for recycling in time and registered properly.
Viewpoint 10: The situation of vaccination units in the jurisdiction should be dynamically grasped and announced.
Article 48 [Conditions for Inoculation Units]
It is suggested to add: each province should publicize the list of vaccination units (including unit name, address and contact number) on the website, and update it at least once every quarter.
Viewpoint 11: change the concept, not to complete the work, but to provide services.
Forty-ninth [vaccination responsibility area] vaccination units shall undertake the vaccination work of the national immunization program in the responsible area, and accept the technical guidance of the local disease prevention and control institutions and the supervision of the health administrative department.
The vaccination unit should undertake the vaccination of all vaccines, not just the national immunization program vaccine (that is, the national free vaccine). In addition, we should change our ideas and regard vaccination as a basic public health service for the public, so as to form a sense of serving people, instead of completing a cold job that must be completed by superiors.
It is suggested that this article be changed to: The vaccination unit shall provide vaccination services for the people in its jurisdiction and accept …
Viewpoint 12: It is the most appropriate for maternity hospitals to issue vaccination certificates.
Article 53 [Vaccination Certificate System] The State practices a vaccination certificate system for children. Within one month after the child is born, his guardian shall go to the vaccination unit where the child lives to undertake vaccination work to apply for a vaccination certificate for him.
The idea of applying for vaccination certificate after one month is quite outdated. There are several problems in this practice: first, the vaccination record is secondary registration, which is easy to make mistakes. Obstetric institutions are registered on specific documents, which are taken to the inoculation unit in the place of residence by parents and copied on the inoculation certificate; Second, the vaccination certificate is not the original vaccination record, and the legal evidence is not strong enough; Third, it is impossible to play the propaganda role of vaccination certificate.
At present, the hospital delivery rate of children in China is very high, and obstetric institutions are also officially recognized vaccination units. The BCG vaccine and the first dose of hepatitis B vaccine vaccinated in obstetric institutions after birth should be registered on the vaccination certificate issued by obstetric institutions.
Shanghai, Hebei, Guangdong and other places have fully realized that maternity institutions have issued vaccination certificates and registered vaccines for newborns. There are a lot of knowledge and instructions on the vaccination certificate in Shanghai, which can be used as publicity materials to provide information for parents, rather than just recording vaccination information.
Viewpoint 13: Children who miss vaccination must be able to attend nursery school.
Article 54 [Inspection of Vaccination Certificate] When children are enrolled in kindergartens and schools, nursery institutions and schools should check the vaccination certificate. If children who have not been vaccinated according to the national immunization plan are found, they should report to the vaccination unit where the children live, and cooperate with the vaccination unit to urge their guardians to replant in the vaccination unit in time after the children are enrolled in kindergartens and schools, unless they cannot be vaccinated for medical reasons.
Many professionals and the public mistakenly believe that missed vaccines are not allowed to enter kindergarten. This article emphasizes that missed vaccines should be replanted after entering kindergarten, which is very good, but it is suggested to emphasize that children should not be restricted from entering kindergarten because of missed vaccines.
Viewpoint 14: Respect science and take public health as the first goal.
Article 90 [Punishment for Illegal Inoculation] If the inoculation personnel violate the vaccination norms during the inoculation process, causing serious consequences, the health administrative department at or above the county level shall give a warning or order them to suspend their practice activities for more than six months but less than one year; If the circumstances are serious, his practice certificate shall be revoked; If a crime is constituted, criminal responsibility shall be investigated according to law.
The key to this clause is how to define the violation of [vaccination norms].
Vaccination norms include normative documents with different sources and authorities. Take influenza vaccination norms as an example:
Egg allergy is taboo in the instructions of most influenza vaccines, but the latest influenza vaccine guide document of China CDC points out that the 2015 edition of Pharmacopoeia has cancelled the taboo of egg allergy, and foreign studies have also proved that egg allergy is not a contraindication for influenza vaccine inoculation.
In other words, the taboo in the manual is set incorrectly. So, can the vaccination doctor vaccinate people who are allergic to eggs with flu vaccine?

At present, there are a large number of clauses with China characteristics lacking scientific basis in China’s vaccination standards. The general starting point of these clauses is to avoid getting into trouble for yourself, not to put public health first. If such vaccination norms are taken as the basis of punishment, it will lead to the tragedy in Dying to Survive.
Personally, I suggest that this excessively severe penalty be abolished. In reality, if there is a vaccination error or accident, the parties will be punished to some extent, and there is no need to increase the penalty.
Authors: Vaccine and Science, Tao Lina, a vaccine expert, a member of China Medical Media Alliance, and an author of er interesting science in medical education.
Disclaimer: My medical science is used to guide the public to establish a scientific understanding of health and avoid being misled by mistakes and rumors. The contents of popular science are personal opinions, for reference only, and have nothing to do with my employer. If my popular science content is wrong, please leave a message and tell me. I am very willing to verify and correct it. Some pictures are from the Internet. If your copyright is involved, please contact me to delete them.